
Center for Mouse Genome Modification (CMGM)
Contacts
Services & Rates

Consultation
The Center for Mouse Genome Modification provides a comprehensive service to generate, maintain and archive genetically modified mouse strains. Investigators and trainees are welcome to discuss their needs and proposed projects including generation of novel mouse strains by CRISPR/Cas9-mediated editing of mouse embryos, gene targeting in ES cells, or transgenic mice by pronuclear microinjection. We provide consultation to accommodate the specific needs and nature of each project. Our services include preparation of CRISPR specific reagents, targeting vectors, transgene constructs, and manipulation of early mouse embryos or ES cells to generate novel mouse strains. We will develop simple PCR genotyping assays as well as the subsequent breeding, management and cryopreservation of various mouse lines.
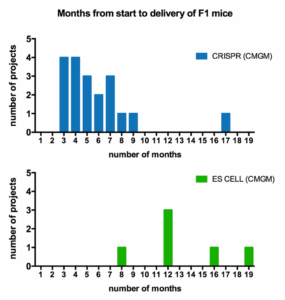
Design and Generation of Genetically Modified Mice by CRISPR and ES Cell Technology
The decision to use CRISPR or ES cells to generate genetically modified mouse strains depends largely on the genomic structure of the gene of interest and the nature of the specific modification such as KI, KO, cKO or cKI etc. In general CRISPR is faster and less expensive, so it is the first choice when possible. Most CRISPR projects take 3-7 months to obtain F1 mice, whereas ES cell projects typically take a year or more.
CRISPR
We have successfully produced different novel mouse strains with gene specific KO, KI (insertions of DNA fragments up to 9kb), point mutations, precision deletions (removal of specific introns), and other novel mouse strains using CRISPR mediated gene editing directly in early mouse embryos.
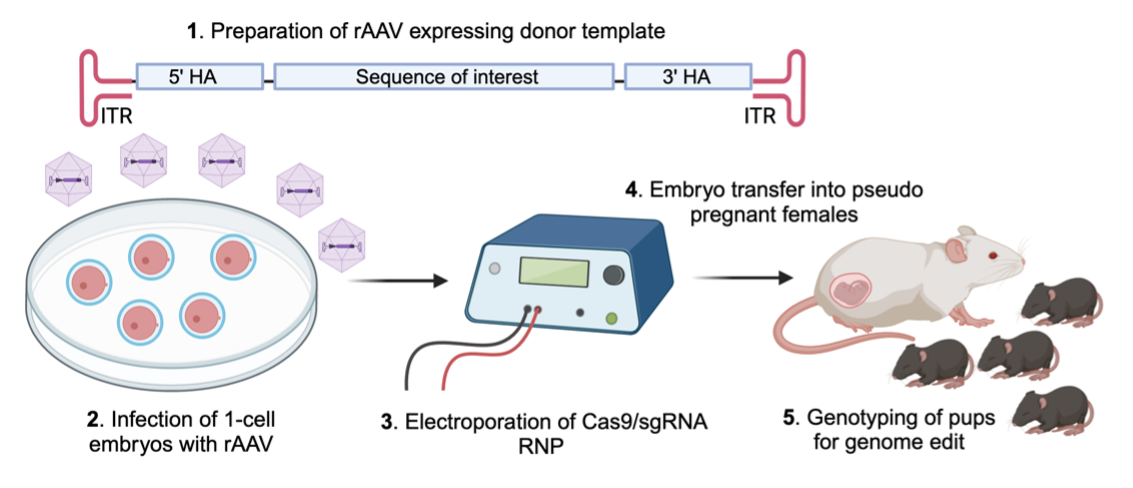
Genome Editing Services Using Recombinant Adeno-Associated Viruses (rAAV)
AAV (adeno-associated virus), which is a single-stranded DNA (ssDNA) virus, can effectively penetrate the zona pellucida and infect one-cell embryos. Recombinant AAV (rAAV) contains the sequence of interest together with 5’- and 3’-homology arms for CRISPR-mediated gene editing in the mouse genome. To this end, we infect one-cell embryos with rAAV followed by electroporation of with Cas9/sgRNA ribonucleoprotein (RNP) for subsequent genome modification in the mouse. The advantage of this approach is that rAAV infection is faster and less laborious than traditional pronuclear microinjection to transduce ssDNA template into mouse embryos. Furthermore, ssDNA template in the form of rAAV genome is a more efficient template for homology-directed repair as compared to double-stranded DNA. This approach significantly increases the efficiency to generate novel mouse models containing the sequence of interest.
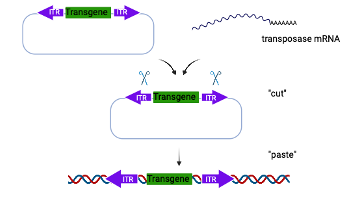
Piggy Bac Mediated Transgenesis
Piggy Bac mediated transgenesis offers many advantages over traditional transgenics. It has a large cargo capacity (up to 200 kb) allowing insertion of large promoters and regulatory elements. The insertion is footprint free and does not leave behind vector sequences. The transgene is also inserted in the host genome in a precise manner as a single copy without any chromosomal rearrangement or deletion (chromothripsis) and therefore offers reliable and consistent transgene expression.
In the Piggy Bac approach, the donor template includes the transgene of interest flanked by inverted terminal repeat (ITR) sequences. The donor template is microinjected into 1-cell embryos along with mRNA encoding the transposase, which integrates the transgene into the host genome through a “cut and paste” mechanism.
Transgenic mice by Pronuclear Microinjection
We provide a service to generate transgenic mice by pronuclear microinjection using one-cell embryos from different genetic backgrounds including C57BL/6j, FVB or CD1. We can prepare the transgene construct as an extra service or use construct supplied by the investigator.
Design and Generation of Targeting Constructs Using Recombineering-based Method
We design and generate gene targeting constructs. The constructs generated in the Center for Mouse Genome Modification (CMGM) include conventional knock-out, knock-in, conditional knock-out, knock-in subtle mutations such as a point mutation and small deletion, and other custom made constructs.
The constructs are derived from C57Bl/6 genomic DNA, unless investigator provides the BAC clones from other strains. We use C57Bl/6 x 129SVEV F1 hybrid ES cell lines for targeting.
Investigators who plan to make a transgenic/targeting construct please contact the CMGM at gttf@uchc.edu.
We will need the construct purpose- conventional or conditional knock-out, point mutation or deletion as well as the MGI number or Genbank accession number.

Import Mouse Lines by Embryo Transfer
When you import mice from other institutions, instead of shipping mice you can ask them to ship mouse embryos in a vial by FedEx. The following is the protocol of shipping embryos for mice providers:
- Superovulate donors and collect E3.5 blastocycts.
- Equilibrate ES media without LIF in a CO2 incubator for at least two hours.
- Fill a cryovial with the ES media (without LIF) to the top to avoid trapping air in the vial.
- Transfer the embryos to the cryovial and seal the vial with parafilm.
- Send by FedEx in an envelope with bubble wrap when weather is mild. In winter, warm ice packs in incubator overnight at 37°C. Then put pre-warmed ice packs in a styrofoam container to make a small incubator for embryos.
- Inform the CMGM when the donors are superovulated, so that we can prepare the embryo recipients.
In Vitro Fertilization
Mouse In Vitro Fertilization (IVF) can be used to rapidly expand mouse lines from a few males that carry the desired genotype or to maintain strains with poor breeding efficiency. However, the success of IVF is dependent on the number of motile sperm that can be retrieved. This is often unknown until after the male mouse is euthanized, the sperm collected from cauda epididymides, and the sperm number and motility assessed under the microscope.
Mouse Colony Management
The CMGM offers mouse colony management as a service to maintain your specific mouse line. The CMGM facility is maintained at level zero containment. All mice enter the facility have to come from an approved vendor or via rederivation. We use a purpose built FileMakerPro program to document all mouse lines housed inside our facility. You will have access to the status of your specific mouse line through the UCH intranet.

Mouse Embryo Cryopreservation
Cryopreservation of your genetically modified mouse strains will help save housing cost for lines that are not actively used for experiments. More importantly, cryopreservation of precious mouse lines will help prevent genetic drift, minimize the impact of disease outbreak and protect against unanticipated disasters and events that lead to cessation of breeding or sudden loss of the line. While embryo cryopreservation is more costly than sperm cryopreservation, it is a more reliable way to archive mouse lines and much easier and less costly to subsequently revive the mouse line. To recover a line, it is a simple thaw and embryo transfer into a pseudopregnant recipient. Please contact the CMGM for details on the process of superovulation/mating/harvesting of your mouse line.
PCR Genotyping
The Center for Mouse Genome Modification offers PCR genotyping services for a fee. The researcher must provide genotyping protocols.
Rederivation of Contaminated Mouse Lines
Female donors will be superovulated to increase the yield of unfertilized oocytes and then set up in mating with males to generate fertilized embryos. We prefer to use wt B6 females around 4 weeks old and males between 2-6 months old as they respond well to superovulation. 1-cell embryos at 0.5 dpc will be harvested and cultured overnight to 2-cell stage. They will then be surgically transplanted into a pseudo-pregnant recipient.
If the line is homozygous, please plan to have at least 4 females and 4 males so we can do the rederivation in one session, otherwise we will have to mate the mice and thus you will incur extra cage costs.
Campus Address
Mailing Address
263 Farmington Avenue, MC 3001
Farmington, CT 06030-3001