
Clinical Core
Contacts
Services

Study Coordination and Management
- Screening/recruitment
- Informed consent process
- Study visits
- Subject Teaching
- Phlebotomy/specimen collection
- Investigational drug administration
- Special testing/procedures
- Other data collection (e.g. questionnaire administration, assistance with self-administered computerized questionnaires)
- Study coordination
- IRB submissions
- Regulatory binder creation/maintenance
- SAE/AE tracking and reporting
- Case Report Form (CRF) design
- Research record chart assembly and maintenance
- Medical exam room use
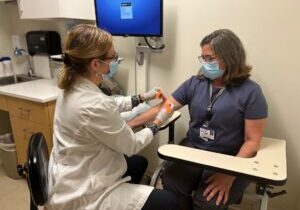
Phlebotomy/Specimen Collection

Informed Consent Process
Location
CRC is located in the main lobby of the Connecticut Tower, just past the main Information Desk. Our outpatient facility includes a reception and waiting area, seven exam rooms (one negative pressure), lab specimen processing laboratory (with countertop refrigerated centrifuge and -80°C freezer), dirty utility room, two in-suite bathrooms and staff office space.
Campus Address
Clinical Research Center, CM 219
UConn Health
Mailing Address
UConn Health
263 Farmington Avenue
Farmington, CT 06030-3805